(Note: cette page n'existe qu'en anglais - Nota: deze pagina bestaat enkel in het Engels)
This document is only a guide to help the users of contained use facilities to be familiar with the regulatory requirements for shipping of infectious substances which are a part of the transportation of dangerous goods regulations. This document is not meant to be a substitute for them. Any transport of biological and infectious substances remains the full and sole responsibility of the sender.
Transport within the facility
To avoid accidental leakage or spillage when moving biological products and infectious substances inside a facility, the transport has to fulfill some criteria, outlined in these documents in French or Dutch.
National and international transport
The following guidelines provide an overview of the transport requirements within the country and across international borders for infectious substances including pathogens, GMOs and biological waste. They provide information for classifying these substances for transportation and ensuring their safe packaging and correct labelling.
- Regulations
- Definitions
- Classification
- Identification
- Packaging
- Marking and Labelling
- Documents
- In summary
- Medical and clinical waste
- Responsibilities
- Annexes (opens in a new page)
1. Regulations
Transport of (potentially) infectious materials is subject to strict national and international regulations. These regulations describe the proper use of packaging materials, as well as other shipping requirements with the aim to reduce the likelihood that packages will be damaged and leak, and thereby prevent accidental exposure to personnel who may handle the material during its shipment.
International transport regulations
United Nations
Regulations for the transport of infectious materials are based upon the United Nations Model Regulations on the Transport of Dangerous Goods (Orange book). These are revised biannually and concern the transport by road, rail, sea or air of dangerous goods, including biological substances.
WHO
The Guidance on regulations for the Transport of Infectious Substances provides practical guidance to facilitate compliance with current international regulations for the transport of infectious substances and patient specimens by all modes of transport.
Transport by air
International Air Transport Association (IATA) Dangerous Goods Regulations. IATA regulation controls the shipment by air of dangerous substances, including biological substances.
IATA regulations follow International Civil Aviation Organization’s (ICAO) Technical Instructions for the Safe Transport of Dangerous Goods by Air as a minimal standard, but may impose additional restrictions.
Transport by road
European Agreement concerning the International Carriage of Dangerous Goods by Road (ADR Agreement). This agreement is regularly amended and updated since its entry into force. It contains the conditions under which dangerous goods may be carried internationally by road in safe conditions.
Transport by rail
The Convention concerning the International Carriage by Rail applies in Europe, the Maghreb and in the Middle East. This convention defines uniform rules concerning the transport of dangerous goods by rail (RID).
Transport by boat
The International Maritime Dangerous Goods Code (IMDG) was formed to prevent all types of pollutions at sea. The code also contains packaging regulations to ensure safe transportation of the goods, and is applicable for all cargo-carrying ships around the world.
Transport by Post
Only infectious substances assigned to category B are accepted for carriage by post.
The Parcel Post Manual which includes the provisions of the Universal Postal Convention relating to postal parcels of the Universal Postal Union (UPU) lays down detailed regulations for the transport of biological substances by post/mail.
Remark: International modal regulations are not intended to supersede any local or national requirements. However, in situations where national requirements do not exist, international modal regulations should be followed. It is important to note that international transport of infectious substances is also dependent on and subject to national import/export regulations.
Specific Belgian legislation
Transport of dangerous goods must comply with the requirements of the various international agreements according to the mode of transport (see above). Furthermore, for EU member States, the transport by road, rail and inland waterways must comply with the requirements of the Directive 2008/68/EC.
In Belgium, the Federal Public Service Mobility and Transport is responsible for the RID (rail), the IMDG Code (boat), the ICAO Technical Instructions (air) and for ADR multimodal aspects of transport (including road) (contact: Annex 1)
The Regions are responsible for the ADR (road) and inland waterways transport. (contact: Annex 2)
A royal decree on postal service (Arrêté royal portant réglementation du service postal du 27/04/2007) defines the conditions for sending perishable biological material, including diagnostic specimens.
2. Definitions
Biological products: Biological products are those products derived from living organisms which are used either for prevention, treatment, or diagnosis of disease in humans or animals, or for development, experimental or investigational purposes related thereto. They include, but are not limited to, finished or unfinished products such as vaccines.
Cultures: Cultures are the result of a process by which pathogens are intentionally propagated. This definition does not include human or animal patient specimens.
GMO: Genetically modified (micro)-organisms. They are microorganisms and organisms in which genetic material has been purposely altered through genetic engineering in a way that does not occur naturally.
Infectious substance: For the purposes of transport, infectious substances are defined as substances which are known or are reasonably expected to contain pathogens. Pathogens are defined as microorganisms (including bacteria, viruses, rickettsia, parasites, fungi) and other agents such as prions, which can cause disease in humans or animals.
Medical or clinical wastes: Medical or clinical wastes are derived from the medical treatment of animals or humans or from bio-research.
Pathogens: Pathogens are defined as micro-organisms (including bacteria, viruses, parasites, fungi and other agents such as prions) which can cause disease in humans or animals.
Patient specimens: These are human or animal materials, collected directly from humans or animals, including, but not limited to, excreta, secreta, blood and its components, tissue and tissue fluid swabs, and body parts being transported for purposes such as research, diagnosis, investigational activities, disease treatment and prevention.
3. Classification
Classification is the process of defining the materials to be carried and of determining whether these materials are regulated Dangerous Goods or not. For these reasons, proper classification of biological materials is a crucial step before their transportation.
UN transport regulations assign dangerous substances to one of nine classes according to the hazard or the most predominant of the hazards they present. Some classes are further divided into divisions.
UN classification of dangerous goods:
|
UN Class |
Dangerous Goods |
Divisions if applicable |
Classification |
|
1 |
Explosives |
1.1 - 1.6 |
Explosive |
|
2 |
Gases |
2.1 |
Flammable gas |
|
2.2 |
Non-flammable, non-toxic gas |
||
|
2.3 |
Toxic gas |
||
|
3 |
Flammable liquid |
|
Flammable liquid |
|
4 |
Flammable solids |
4.1 |
Flammable solid |
|
4.2 |
Spontaneously combustible substance |
||
|
4.3 |
Substance which emits flammable gas in contact with water |
||
|
5 |
Oxidizers and organic peroxides |
5.1 |
Oxidising substance |
|
5.2 |
Organic peroxide |
||
|
6 |
Toxic and infectious substances |
6.1 |
Toxic substance |
|
6.2 |
Infectious substance (Annex 3) |
||
|
7 |
Radioactive material |
|
Radioactive material |
|
8 |
Corrosive substances |
|
Corrosive substance |
|
9 |
Miscellaneous dangerous substances |
|
Miscellaneous dangerous substance (Annex 4) |
Infectious substances are classified into Division 6.2
The infectious substances class includes pathogens but also cultures, biological products and patient specimens which are known or reasonably believed to contain infectious substances.
Infectious substances are further divided in categories A and B:
> Category A
An infectious substance which is transported in a form that, when exposure to it occurs, is capable of causing permanent disability, life-threatening or fatal disease in otherwise healthy humans or animals.
Note : An exposure occurs when the infectious substance is released outside of the packaging, resulting in physical contact with humans or animals.
Indicative examples of infectious substance included in Category A are listed in a table in the UN Model Regulations (Annex 8).
> Category B
An infectious substance that does not meet the criteria for inclusion in Category A.
> Exceptions
- Substances in a form that any pathogens have been neutralized or inactivated such that they no longer pose a health risk.
- Environmental samples (including food and water samples) which are not considered to pose a significant risk of infection.
- Dried blood spots (onto absorbent material).
- Faecal occult blood screening samples.
- Blood or blood components to be used for transfusion or transplantation.
- Human or animal specimens for which there is minimal likelihood that pathogens are present
GMOs which are not infectious are classified into Class 9.
4. Identification
After classification, dangerous goods are assigned UN numbers and proper shipping names according to their hazard and their composition. Proper shipping names are used to clearly identify the dangerous article or substance.
UN numbers and proper shipping names:
|
UN class or division |
UN Number |
Proper shipping name |
|
6.2 - Category A |
UN 2814 UN 2900 |
Infectious substance, affecting humans Infectious substance, affecting animals only |
|
- Category B |
UN 3373 |
Biological substance, Category B |
|
9 |
UN 3245 |
Genetically modified organisms or Genetically modified microorganisms |
5. Packaging
Infectious substances shall be packed in such a manner that they arrive at destination in good condition and present no hazard during transport.
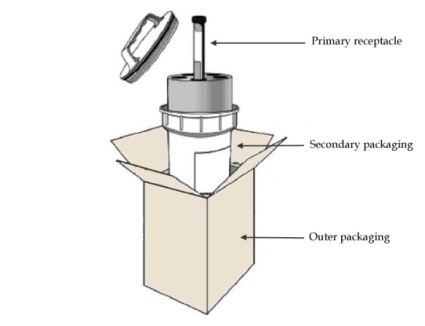
Basic triple packaging system
All infectious substances shall be triple packed. General requirements for a triple packaging system are as follows:
The packaging shall be of good quality, strong enough to withstand the shocks and loadings normally encountered during transport. Packaging shall be constructed and closed so as to prevent any loss of contents that might be caused under normal conditions of transport, by vibration, or by changes in temperature, humidity or pressure.
The components of a triple packaging are:
- Primary receptacle: The primary receptacle containing the specimen shall be watertight, leakproof. The primary receptacle is wrapped in enough absorbent material to absorb the entire contents in case of breakage or leakage.
- Secondary packaging. A second watertight, leakproof packaging is used to enclose and protect the primary receptacle(s). Several wrapped primary receptacles may be placed in a single secondary packaging.
- Outer packaging. The secondary packaging is placed in outer shipping packaging with suitable cushioning material. Outer packaging protects their contents from outside influences, such as physical damage.
Packaging instructions
Although the basic triple packaging system is required for all transport, the packaging requirements are increasingly stringent in proportion to the hazards of the transported substance, therefore they are based directly on the classification of the material to be carried.
These requirements are determined by the UN regulations in the form of Packaging Instructions (PI). To each UN number and proper shipping name corresponds a PI.
|
UN class or division |
UN Number |
Proper shipping name |
Packing instruction |
|
6.2 - Category A |
UN 2814 UN 2900 |
Infectious substance, affecting humans Infectious substance, affecting animals only |
|
|
- Category B |
UN 3373 |
Biological substance, Category B |
|
|
9 |
UN 3245 |
Genetically modified organisms or Genetically modified microorganisms |
Overpack
Several packages could be combined into a single enclosure for convenience of handling and stowage during carriage. The enclosure to be sent by a single shipper to the same destination is called overpack. An overpack shall be marked with the word “overpack”.
6. Marking and Labelling
Marking and labelling on packaging are the main source of information for any person handling the shipment. It is therefore important to properly mark and label the packaging to provide information about the contents of the package, the nature of the hazard, and the packaging standards applied, so that it is handled with safe procedures
All markings and labels must be placed on the packages or overpacks in such a place and such size that they are readily visible.
> Marking
Packaging markings for Category A Infectious substances include:
- Proper shipping name and identification number preceded by the letter “UN”
Example: Infectious substance, affecting human
UN 2814 - Name and address of shipper and consignee,
- Name and telephone number of a responsible person,
- Net quantity of infectious substances in each package,
- Hazard labels
- If applicable: handling labels
- When dry ice is used: Net quantity of dry ice, “Dry Ice, UN 1845”
Packaging markings for Category B Infectious substances include:
- Proper shipping name: Biological Substance, category B
- A mark with UN number as follows:

- Name and address of shipper and consignee,
- Name and telephone number of a responsible person (if not shown on the air waybill),
- If applicable: Hazard and handling labels
- When dry ice is used: Net quantity of dry ice, “Dry Ice, UN 1845”
Packaging markings for GMOs & GMMs include:
- A mark with UN number as follows:

-
Name and address of shipper and consignee.
> Labelling
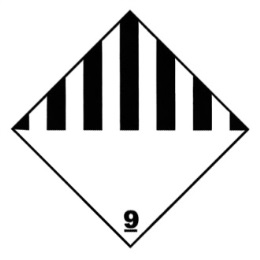
Hazard labels:
- Division 6.2, Infectious substances, Category A:

- Class 9, GMO, dry ice:
Handling labels:
- Package orientation. To use if more than 50 ml of liquid in primary receptacle.

- Cargo aircraft only. Some dangerous goods must not be carried on an aircraft with passengers.

- Cryogenic liquids. To use with a Non-Flammable Gas label when shipping cryogenic materials by air.

7. Documents
For UN2814 and UN2900 (P620), a shipper’s declaration for dangerous goods (DGD) must be completed as well as an itemized list of contents that shall be enclosed between the secondary packaging and the outer packaging.
The proper shipping name must be supplemented with the technical name (e.g. Bacillus anthracis) on the shipper’s declaration. Technical names do not need to be shown on the package.
Example of a filled DGD for a shipment of Category A Infectious substance (click for lager):
An air waybill, issued by the transporter, is also needed for air transport. Equivalent documents exist for road, rail and sea transport.
8. In summary
Flowchart for the classification of infectious materials for transport (click for lager):
9. Medical and clinical waste
Medical or clinical wastes are wastes derived from the veterinary treatment of animals, the medical treatment of humans or from bio-research. Medical or clinical wastes belong to division 6.2, infectious substances.
- Medical or clinical wastes containing Category A infectious substances shall be assigned to UN 2814 or UN 2900 as appropriate. They shall be packed as Category A infectious substances following packing instruction P620.
Solid medical wastes containing Category A infectious substances generated from the medical treatment of humans or veterinary treatment of animals may be assigned to UN 3549. They shall be packed following packing instruction P622. The UN 3549 entry shall not be used for waste from bio-research or liquid waste.
- Medical or clinical wastes containing Category B infectious substances (or which have a low probability of containing infectious substances) shall be assigned to UN 3291 and packing group II (medium hazard).The proper shipping name is “Clinical waste, unspecified, N.O.S.” or “(Bio) medical waste, N.O.S. or “Regulated medical waste, N.O.S.” (N.O.S. = Not Otherwise Specified).
The transport of UN 3291 by road shall meet the requirements of the packing instruction P621:
Wastes of UN 3291 could be carried within drums, boxes and jerricans which are strong enough to arrive in good condition at destination and present no hazard during transport. For large quantities of waste, bulk containers or combination packages consisting of an outer and an inner packaging such as a bag and plastic container could be used.
In all cases, the outer packaging shall be rigid, leakproof and contain absorbent material in sufficient quantity to absorb all the liquid that may be present. It shall bear the UN specification marking, which indicates that the packaging is UN-approved.
Wastes of UN 3291 containing sharp objects shall only be carried in UN-approved rigid packagings.
Decontaminated medical or clinical wastes which previously contained infectious substances are not subject to these provisions.
10. Responsibilities
> Responsibilities of the sender
The sender is responsible for checking whether goods can be transported. He must inform the carrier of the exact nature of the danger and indicate, if necessary, the precaution to be taken. For that, he has to:
- Classify any dangerous goods to be transported;
- Package in accordance with UN packaging provisions;
- Mark the shipment with the proper shipping name, UN number;
- Label the shipment according to regulations;
- Provide completed transport documents in compliance with regulations.
> Responsibilities of the transporter
- The transporter must ensure that the shipment has been correctly prepared in accordance with the regulations (by using a control checklist)
- The airline company is responsible for storage, loading, inspection, emergency procedures, reporting incident or accident, archiving of records, training;
- Establish an Air Waybill (AWB)