(Note: cette page n'existe qu'en anglais - Nota: deze pagina bestaat enkel in het Engels)
Contents
- Introduction
- Risk assessment
- Intrinsic properties of cell cultures
- Properties acquired as a result of genetic modification
- Properties acquired as a result of infection with pathogenic agents
- Deliberate infection with pathogens
- Adventitious contaminating agents
- Bacteria and Fungi
- Mycoplasma
- Viruses
- Parasites
- Prion
- Type of manipulation
- Biosafety recommendations and containment measures
- Conclusions
- Further reading and helpful links
- Annexes (opens in a new page)
Introduction
The use of animal and human cell cultures has become very beneficial for diverse applications in biotechnology and biomedical research. Originally used as substrates for the production of viral vaccines, animal and human cell cultures became an indispensable tool to produce a variety of products, including biopharmaceuticals, monoclonal antibodies and products for gene therapy. The use of animal and human cell cultures constitute also adequate test systems for studying biochemical pathways, virus production, pathological mechanisms or intra- and intercellular responses.
Along with the increasing importance of the contained use of animal and human cell cultures, biosafety concerns have pointed to the risks with respect to human health and environmental considerations. A maximal reduction of these risks necessitates a thorough biosafety risk assessment, taking account of the type of manipulation and the biological hazards inherent to the use of cell cultures. The risk assessment should result in the implementation of appropriate containment measures and work practices in order to provide maximal protection of human health and environment.
Risk assessment
Risk assessment of human/animal cell cultures is based on both the intrinsic properties of the cell culture - including subsequent properties acquired as a result of genetic modification(s) - and the possibility that the cell culture may inadvertently or deliberately be contaminated with pathogens. In addition, risk assessment should be performed according to the type of manipulation.
Intrinsic properties of cell cultures
The following intrinsic properties of cell cultures should be considered while performing the risk assessment: source (species of origin), cell types or tissues, culture type.
|
Source (species of origin) |
Cell types or tissues |
Culture type |
|
The closer the genetic relationship of the cell culture is to humans, the higher the risk is to humans since contaminating pathogens usually have specific species barriers. |
Consider the tumour inducing potential of cell types. |
|
|
increasing order of risk
|
increasing order of risk
|
increasing order of risk
|
|
avian and invertebrate cells |
epithelial and fibroblastic cells |
well-characterized cell lines |
|
mammalian cells (other than human or primate) |
gut mucosa |
|
|
non-human primate cells |
endothelium |
|
|
human cells |
neural tissues |
|
|
|
hematopoietic cells |
|
Properties acquired as a result of genetic modification
Recombinant cells may have increased (or decreased) abilities to cause harm to human health and environment compared to their non-recombinant counterparts. The properties that recombinant cells acquire following genetic modification must be determined as well. This includes an evaluation of all events intervening in the process of genetic modification:
- the recipient organism properties (host cell)
- the donor organism properties
- the characteristics and location of the inserted genetic material
- the vector
The Annex of Commission Decision 2000/608/EC provides guidance notes for risk assessment of genetically modified microorganisms.
- see example 1 : transfection of mouse T-lymphoma cells with human interleukin 2 using mammalian expression vector
- see example 2: retroviral packaging cell lines
Properties acquired as a result of infection with pathogenic agents
Deliberate infection with pathogens
Cells may be deliberately infected with pathogens. In this case, the determination of potential hazards related to infected cell cultures requires an examination of the inherent properties of the infecting pathogen. This implies an assessment of a list of criteria, which are specific to the pathogen, along with aspects such as the existence of effective therapies or prophylaxis. An evalutation of these criteria have been used to classify pathogens into classes of biological risk, also called risk groups. The biological risk of infected cell cultures will depend on the biological risk of the infecting pathogen(s).
- see example : culture of bovine leukocytes infected with Theileria parva
Adventitious contaminating agents
When manipulating cell cultures, the presence of adventitious contaminating agents constitutes the main hazard to humans. Contamination may occur by the source (e.g. infected animals or tissues), during cell handling (repeated passages and use in the laboratory) or by using contaminated biological reagents (e.g. media and additives derived from bovine sources are often contaminated with bovine viral diarrhoea virus, BVDV).
Bacteria and Fungi
In general bacterial or fungal contamination can be readily detected in cell cultures because of their capacity to overgrow cell cultures. Bacterial and fungal infections are relatively easy to prevent and to treat.
Mycoplasma
Compared to bacterial or fungal infections, contaminations with mycoplasma give more problems in terms of incidence, detectability, prevention and eradication. Mycoplasma infection may go undetected for many passages, causing a variety of unpredictable effects causing harm to the host cell. Mycoplasma infection may also influence the sensitivity of host cells for growth of viruses (Hargreaves et al., 1970). Beside the fact that this intracellular bacteria is one of the most common cell culture contaminants, it should be considered that some of contaminating Mycoplasma spp. belong to class of risk 2. Together with M. arginini, M.orale, M.pirum and M. fermentans, pathogenic organisms like M. gallisepticum (class of risk 3 for animals), M. hyorhinis (class of risk 2 for animals), and M. pneumoniae (class of risk 2 for humans) account for more than 96% of mycoplasma contaminants in cell cultures.
Viruses
Viral contamination needs particular attention because infection may be without cytopathic effect for the cell culture or may be latent (e.g. herpesvirus) and hard to detect. Human and non-human primate cultures are more likely to harbour viruses that are highly pathogenic to humans. Of particular concern are the blood-borne viruses such as Hepatitis B Virus (HBV), Human Immunodeficiency Virus (HIV) and others such as Hepatitis C virus (HCV) and Human T-cell lymphotropic viruses (HTLV). However, non-human cell cultures are not without risks as they may contain viruses with a broader host range able to infect humans such as rodent cell culture carrying hantavirus (Lloyd G & Jones N, 1986) or primate cells harbouring Marburg virus.
- see example : Lymphocytic choriomeningitis virus (LCMV) contamination of murine cell cultures
Parasites
Adventitious contamination with parasites may be an issue when handling freshly prepared primary cell cultures or tissue cultures originating from a donor organism that is known or suspected to be infected with a specific parasite. Well-known intracellular protozoan parasites for which laboratory-acquired infections have been reported are Toxoplasma gondii, Trypanosoma cruzi, Leishmania sp, Cryptosporidium parvum , Plasmodium sp. etc. (reviewed by Herwaldt et al., 2001).
Prions
Though a limited number of cultured cell lines (e.g. mouse neuroblastoma cell lines Sc N2a) have been shown to promote, upon subpassaging, stable and persistent replication of PrP(Sc) as well as infectivity (Solassal J, et al., 2003), most cell lines are resistant to prion infection (Butler, et al., 1888). However, in contrast to most of the infectious agents, prions are particularly difficult to inactivate. In fact no method can guarantee total inactivation of these agents. So, one should bear these considerations in mind when using growth media of bovine origin.
Type of manipulation
In addition to the determination of biological risk of animal cell cultures, the consideration of the type of manipulation constitutes a key aspect in the completion of a thorough risk assessment. Issues regarding the type of manipulation include:
- the characteristics of the environment likely to be exposed
- e.g. mucous membranes of the manipulator may be exposed when handling cell cultures out of a biosafety cabinet type II
- the characteristics of the activity (scale, type of procedure, culture conditions)
- e.g. Altered culture conditions can have significant effects to safe handling of human or animal cell cultures as it may result in altered neoplasia (Stoker et al., 1990), release of endogenous viruses (Cunningham DA et al., 2004) or altered expression of cell surface receptors. Changing culture conditions may thus lead to altered susceptibility of the cultured cells to viruses (Anders et al., 2003; Vincent et al., 2004).
- e.g. human cell cultures that harbour infectious agents can represent lower risks for human health once they are fixed by glutaraldehyde or formaldehyde/aceton for immunostaining or flow cytometry.
- any non-standard operations
- e.g. depletion of primary cells from tissues; manipulations likely to generate aerosols.
As some clinical approaches such as stem cell therapy, gene therapy, xeno- or allotransplantation involve ex vivo cell culturing, many aspects of the risk assessment – as mentioned above- should be applied. However, culturing cells for therapeutic purposes justifies more careful consideration regarding quality, efficacy, safety, ethical, social and regulatory issues that will not be addressed on this webpage. More information can be obtained on clinical trials with GMOs for human or veterinary use.
Biosafety recommendations and containment measures
The examination of biological risks related to animal cell cultures and the consideration of the type of manipulation allows the determination of adequate containment level in order to protect human and environmental health. The set up and implementation of an adequate containment level include a list of general and more specific work practices and containment measures.
- Precautionary measures should be applied whenever handling animal cell cultures. Much of these measures basically aim at reducing the risk of contamination with adventitious agents by ensuring protection of both operator and cell culture.
- Cells are reasonably considered free of adventitious contaminating pathogens if a number of conditions are fulfilled.
- If cell cultures are known to harbour an infectious etiologic agent or virus, containment measures should be the same as that recommended for the etiologic agent.
The flowchart hereunder offers a schematic guidance for the assignment of appropriate containment levels and is based on, but not limited to, key features of risk assessment as outlined above. This flowchart is only indicative and should be applied and /or reconsidered according to case specific conditions and risk assessments.
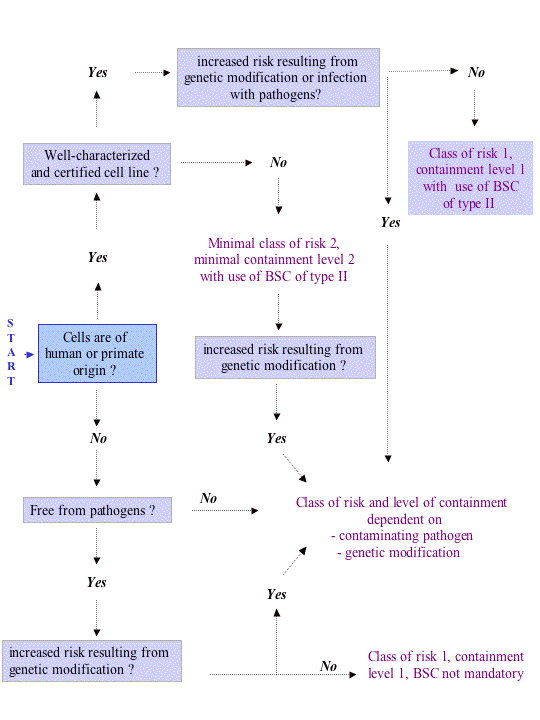
BSC type II = Biosafety cabinet type 2. Contrary to a biosafety cabinet of class I, which only protects the operator and the environment, the use of a biosafety cabinet of class II aims at protecting the operator, the environment and the cell culture, the latter being of importance in order to prevent contamination of cell cultures. In view of this, animal cell cultures should never be manipulated in a biosafety cabinet with horizontal laminar air flow.
Conclusions
The assignment of appropriate containment requirements cannot be generalised, it varies on a case-by-case basis and should rely on a thorough risk assessment, including considerations of intrinsic cell properties (including recombinant properties), potential contamination with pathogens and type of manipulation. Adventitious contaminating agents probably constitute the main hazard associated to the manipulation of cell cultures since they are often difficult to detect and therefore less verifiable.
Further reading and helpful links
Cell culture and cell lines data bases
- ATCC (USA) [link]
- ECACC European collection of cell cultures [link]
- DSMZ German Collection of Microoganisms and Cell cultures [link]
- ICLC Interlab Cell line Collection [link]
- HyperCLDB (hypertext version of the Cell Line Data Base) [link]
- JCRB Japanese Cancer Research Resources Bank [link]
- The University of Michigan Breast Cell Line/Tissue Bank and Data Base [link]
- CABRI Common Access to Biological Resources [link]
Guidelines
- U.K.
- Health Canada
- Laboratory Centre for Disease Control. The Laboratory Biosafety guidelines, 3rd edition-draft, September 20, 2001
- European Union
- EMEA Committee for Human Medicinal Products (CPMP)EMEA guidelines, Position statement on the use of tumourigenic cells of human origin for the production of biological and biotechnological medicinal products. CPMP/BWP/1143/00 [link]
- EMEA. Note for guidance on quality of biotechnological products: viral safety evaluation of biotechnology products derived from cell lines of human or animal origin. CPMP/ICH/295/95 [link]
- Switzerland
- Center for Biosafety and sustainability (BATS) Biosafety of Mammalian Cell Cultures, Proceedings Basel Forum on Biosafety 28 October 1993
References
- Anders M., Hansen R., Ding R.X., Rauen K.A., Bissell M.J., Korn W.M. (2003). Disruption of 3D tissue integrity facilitates adenovirus infection by deregulating the coxsackievirus and adenovirus receptor. PNAS. 100(4), 1943-8.
- Biosafety of virus-derived vectors (2003), guest editor W.Moens, Current Gene Therapy, Vol 3, pp. 495-611.
- Butler D.A., Scott M.R., Bockman J.M., Borchelt D.R., Taraboulos A, Hsiao K.K., Kingsbury D.T., Prusiner SB (1988). Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J. Virol.62(5), 1558-64.
- Cunningham D.A., Dos Santos Cruz G.J., Fernandez-Suarez X.M., Whittam A.J., Herring C., Copeman L., Richards A., Langford G.A. (2004). Activation of primary porcine endothelial cells induces release of porcine endogenous retroviruses. Transplantation. 77(7), 1071-9.
- Drexler H.G., Uphoff C.C., Mycoplasma contamination of Cell Cultures In Sper R.E. ed. "The Encyclopedia of Cell Technology". Wiley, New York, 2000, pp 609-627.
- Doblhoff-Dier O. & Stacey G., Cell lines : applications and biosafety. In Fleming DO & Hunt DL., eds,"Biological safety, principles and practices". 3rd edition, Washington: ASM Press; 2000, pp. 221-39.
- Dull, T. Zuffery, R., Kelly, M. Mandel, R.J., Nguyen, M., Trono, D., and Naldini, L. (1998). A third-generation lentivirus vector with a conditional packaging system. J. Virol., 72, 8463-8471.
- Gillet, L., Minner, F. Detry, B., Farnir, F., Willems, L., Lambot, M., Thiry, E., Pastoret, P.-P., Schynts, F. and Vanderplasschen A. (2004). Investigation of the susceptibity of Human Cell Lines to Bovine Herpesvirus 4 Infection: Demonstration that Human cells Can Support a Nonpermissive Persistent Infection which Protects Them Against Tumor Necrosis Factor Alpha-Induced Apoptosis. J. Virology, 78, pp. 2336-2347.
- Hargreaves F.D. and Leach R.H. (1970). The influence of mycoplamsa infection on the sensitivity of HeLa cells for growth of viruses. J. Med. Microbiol. 3, 259.
- Herwaldt B.L., Laboratory-acquired parasitic infections from accidental exposure. Clin Microbiol Rev. 2001, 14(4), 659-88. Review.
- Lloyd G., Jones N. (1986) Infection of laboratory workers with hantavirus acquired from immunocytomas propagated in laboratory rats. J Infect. 12(2), pp. 117-25.
- Mahy.W., Dykewicz C., Fisher-Hoch S., Ostroff S., Tipple M., Sanchez A. (1991). Virus zoonoses and their potential for contamination of cell cultures. Dev Biol Stand. 75, 183-9.
- Pardal, R. Clarke, M.F., Morrison, S.J. (2003) Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer Dec 3(12), 895-902.
- Pater M.M., Pater A (1985). Human papillomavirus types 16 and 18 sequences in carcinoma cell lines of the cervix. Virology 145, 313-318.
- Pauwels K., Herman P., Van Vaerenbergh B., Do thi C.D., Berghmans L., Waeterloos G., Van Bockstaele D., Dorsch-Häsler K., and Sneyers M.(2007). Animal Cell Cultures: Risk Assessment and Biosafety Recommendations. Applied Biosafety 12(1), 27-39.
- Solassol J., Crozet C., Lehmann S. (2003) Prion propagation in cultured cells. Br Med Bull. 66, 87-97.
- Stacey, G. (1995) Risk assessment of animal cell culture procedures, Biosafety Journal 1(1) (BY95008).
- Stoker A.W., Hatier C., Bissell M.J.(1990). The embryonic environment strongly attenuates v-src oncogenesis in mesenchymal and epithelial tissues, but not in endothelia. J Cell Biol.111(1), 217-28.
- Vincent T., Pettersson R.F., Crystal R.G., Leopold P.L.(2004) Cytokine-mediated downregulation of coxsackievirus-adenovirus receptor in endothelial cells. J Virol. 78(15), 8047-58.

